
Fig. 1 Structure of each fatty acid
Back to Previous Page
4. Studies on the oxidation of oils and their structure
Details
The oxidation of oils occurs in fatty acids that are part of TAGs. Fatty
acids are principally categorized into 3 types, namely, saturated fatty
acids such as octanoic acid (C10:0), palmitic acid (C16:0), and stearic
acid (C18:0); monounsaturated fatty acids such as palmitoleic acid (C16:1n-7)
and oleic acid (C18:1n-9); and polyunsaturated fatty acid such as linoleic
acid (C18:2n-6), alpha-linoleic acid (C18:3n-3), eicosapentaenoic acid
(C20:5n-3, EPA), and docosahexaenoic acid (C22:6n-3, DHA). Polyunsaturated
fatty acids are most susceptible to oxidation.

Fig. 1 Structure of each fatty acid
Figure 2 indicates the structure of DHA, which is mostly susceptible to oxidation among fatty acids. The site of initial oxidation by an active oxygen species is hydrogen that is bound to methylene located between two double bonds (also called "active methylene"). Consequently, a fatty acid with many double bonds, and therefore many active methylene groups, is easily oxidized. EPA and DHA are characteristically found in fish oil and contain many double bonds in their structures. Therefore, fish oil is more susceptible to oxidation than other kinds of oils.
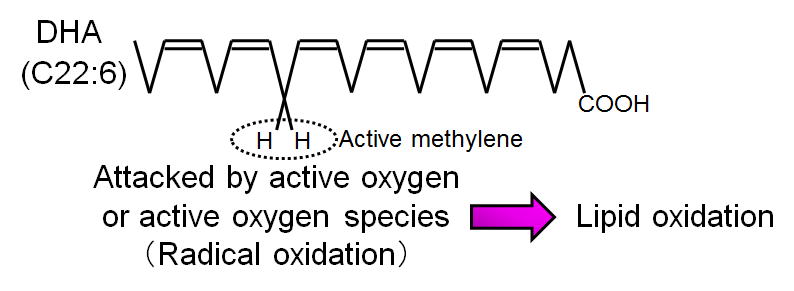
Fig. 2 Oxidized site of polyunsaturated fatty acids
It is also well known, however, that compared to
linoleic acid (when dispersed in water), which contains only one active
methylene, EPA and DHA are barely oxidized when they are dispersed in water.
This phenomenon is very strange because the ability to get oxidized is exactly
inverse when these fatty acids are present in the form of TAGs in oils.
N. Gotoh, Y. Noguchi, A. Ishihara, K. Yamaguchi, H. Mizobe, T. Nagai, I. Otake, K. Ichioka, and S. Wada, Highly unsaturated fatty acid might act as an antioxidant in emulsion system oxidized by azo compound. J. Oleo Sci. 59, 631-638 (2010). [Paper (pdf)]