Back to Previous Page
2. Development of an analytical method for
triacylglycerol isomers

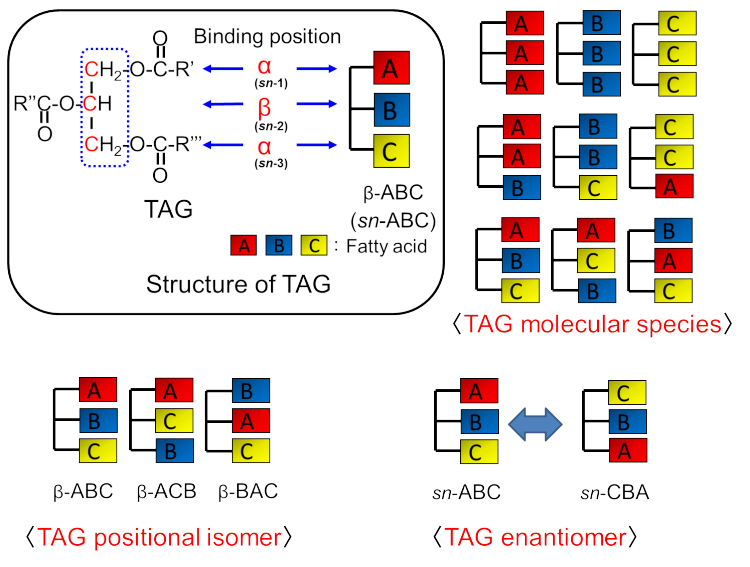
Fig. 1 Binding positions of three fatty acids in TAG and TAG isomers.
Glycerol possesses three alcohol groups, all of which are esterified with
fatty acids to yield TAG. Figure 1 provides options for distinguishing
between isomers in the case of the three kinds of fatty acids denoted as
fatty acids “A”, “B” and “C.”
TAG molecular species
When the combination of three fatty acids bound to
glycerol differs, each TAG is distinguished as a “TAG molecular species.” This approach
has been used to evaluate the adulteration of
olive oil.
TAG positional isomer
The glycerine backbone has two types of alcohol groups.
A primary alcohol group is located at the ends of the glycerine molecule,
whereas the secondary alcohol is located on the central carbon atom of the
molecule. The positions of the primary alcohol group and secondary alcohol
group are often named as the a position
and b position, respectively. The examples of TAG
positional isomers indicated in Fig. 1 are b-ABC (left), TAG-binding fatty acid “B” (b position), b-ACB
(center), TAG-binding fatty acid “C” (b position),
b-BAC (right), and TAG-binding fatty acid “A” (b position). These TAGs derived from the same fatty acid
species; however, the fatty acid binding at the b position is different. Consequently, these TAGs are considered
asTAG positional isomers.
When fats and oils are digested in the human intestine,
pancreatic lipase hydrolyzes fatty acids locating at the a position only; thus, the a and b positions
of TAG can be distinguished.
TAG enantiomer
In a TAG molecule that consists of three different
fatty acids, the secondary carbon atom of glycerine becomes an asymmetric carbon atom. Consequently, the two a positions must be distinguished as sn-1 and sn-3 positions. The beta position is automatically called the sn-2 position. An example of TAG
enantiomers has also been indicated in Fig. 1, where both TAG molecules also
have fatty acids “A” and “C” at the a positions.
However, the two a positions
can be distinguished as sn-1 and sn-3 positions and the TAG molecules can
be considered as enantiomers.
During TAG synthesis in the human body, fatty acid esterification initially occurs at the sn-1 position and later at the sn-2 position. Finally, fatty acid esterification occurs at the sn-3 position, leading to TAG formation. During this process, the body can
also distinguish between these three positions.
At our laboratory, we have developed methods for separation and analyzing
these TAG isomers by HPLC. A detailed description of these methods has
been provided in the papers shown below.
F. Beppu, T. Nagai, K. Yoshinaga, H. Mizobe, K. Kojima, and N. Gotoh, Quantification
of triacylglycerol molecular species in cocoa butter using high-performance
liquid chromatography equipped with nano quantity analyte detector. J. Oleo Sci. 62, 789-794 (2013). [Paper (pdf)]
K. Yoshinaga, T. Nagai, H. Mizobe, K. Kojima, and N. Gotoh, Simple method
for the quantification of milk fat content in foods by LC-APCI-MS/MS using
1,2-dipalmitoyl-3-butyroyl-glycerol as an indicator. J. Oleo Sci. 62, 115-121 (2013). [Paper (pdf)]
N. Gotoh, Y. Matsumoto, T. Nagai, H. Mizobe, K. Yoshinaga, K. Kojima, I.
Kuroda, Y. Kitamura, T. Shimizu, H. Ishida, and S. Wada, Actual ratio of
triacylglycerol positional isomers in milk and cheese. J. Oleo Sci. 61, 173-180 (2012). [Paper (pdf)]
N. Gotoh, Y. Matsumoto, T. Nagai, H. Mizobe, I. Otake, K. Ichioka, I. Kuroda,
H. Watanabe, N. Noguchi, and S. Wada, Actual ratios of triacylglycerol
positional isomers consisting of saturated and highly unsaturated fatty
acids in fishes and marine mammals. Food Chem. 127, 467-472 (2011). [Abstract]
T. Nagai, H. Mizobe, I. Otake, K. Ichioka, K. Kojima, Y. Matsumoto, N.
Gotoh, I. Kuroda, S. Wada, Enantiomeric separation of asymmetric triacylglycerol
by recycle high-performance liquid chromatography with chiral column. J. Chromatgr. A, 1218 ,2880-2886 (2011). [Abstract]
T. Nagai, N. Gotoh, H. Mizobe, K. Yoshinaga, K. Kojima, Y. Matsumoto, and
S. Wada, Rapid separation of triacylglycerol positional isomers binding
two saturated fatty acids using octacocyl silylation column. J. Oleo Sci. 60, 345-350 (2011). [Paper (pdf)]
N. Gotoh, S. Wada, and T. Nagai, Separation of asymmetric triacylglycerols
into their enantiomers by recycle high-performance liquid chromatography.
Lipid Tech. 23, 105-108 (2011). [Abstract]
N. Gotoh, Y. Matsumoto, H. Yuji, T. Nagai, H. Mizobe, K. Ichioka, I. Kuroda,
N. Noguchi, and S. Wada, Characterization of non-endcapped polymeric ODS
column for the separation of triacylglycerol positional isomers. J. Oleo Sci. 59, 71-79 (2010). [Paper (pdf)]
N. Gotoh, Y. Noguchi, A. Ishihara, K. Yamaguchi, H. Mizobe, T. Nagai, I.
Otake, K. Ichioka, and S. Wada, Highly unsaturated fatty acid might act
as an antioxidant in emulsion system oxidized by azo compound. J. Oleo Sci. 59, 631-638 (2010). [Paper (pdf)]